Manual cervical cytology workflows are struggling to keep pace with increasing screening volumes, workforce shortages, and growing pressure for consistent, timely results.
The World Health Organization has set an ambitious global target, the 90-70-90 goals by 2030, with the aim of eliminating cervical cancer as a public health problem (defined as ≤4 cases per 100,000 women) by achieving:
If labs aren’t digitally equipped to accurately triage, quantify, and standardize screening at scale, these 2030 targets, and the broader goal of reducing cervical cancer incidence globally, will remain out of reach.
CytoSiA is a complete digital cytology solution purpose-built to scale cervical cancer screening.
It combines Brightfield slide scanning, AI-powered triage, and automated reporting into a seamless, scalable workflow that helps labs deliver faster, more consistent results, without costly infrastructure or locked platforms.
Whether it is a routine diagnostic* lab, regional center, national screening program, or mobile diagnostic* outreach clinic, CytoSiA makes cervical cytology screening more efficient, accessible, and actionable, even in low-resource settings with limited on-site expertise.
Whether it is a routine diagnostic* lab, regional center, national screening program, or mobile diagnostic* outreach clinic, CytoSiA makes cervical cytology screening more efficient, accessible, and actionable, even in low-resource settings with limited on-site expertise.
Scan. Index. Analyze.
A seamless digital workflow from slide to structured report.
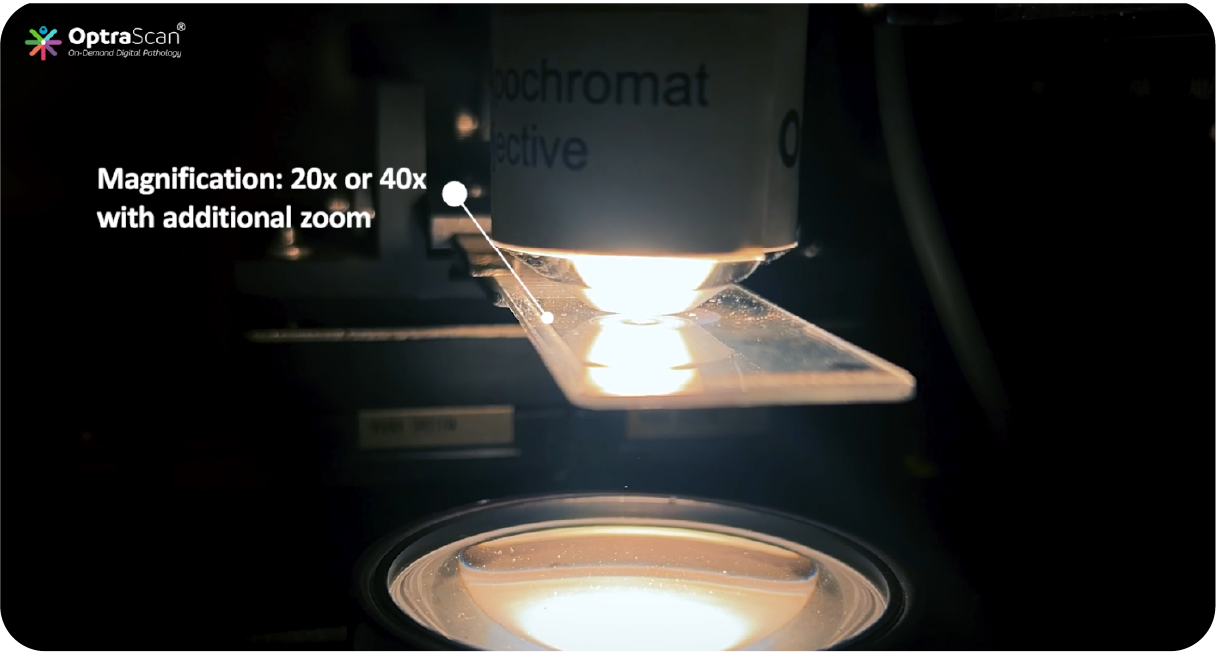
Scanning Options:
Indexing & AI-Triage:
Cell Classification Report:
CytoSiA is mobile-ready and cloud-connected, enabling programs to extend cervical cancer screening into communities that need it most.


Please fill out the form below with information related to your area of interest.
You will be contacted by one of OptraSCAN representatives shortly.
Please complete all questions with an asterisk (*)